Understanding the photophysical properties of rhenium(i) compounds coordinated to 4,7-diamine-1,10-phenanthroline: synthetic, luminescence and biological studies†
Abstract
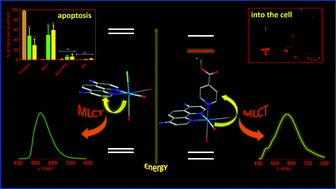
In the present study, the photophysical properties and preliminary time-dependent density functional theory (TD-DFT) data of new rhenium(I) polypyridyl compounds, fac-[Re(L)(Am2phen)(CO)3]0/+, where Am2phen = 4,7-diamine-1,10-phenanthroline and L = Cl and ethyl isonicotinate (et-isonic), provided new insights into excited-state deactivation through an unusual inversion between two metal-to-ligand charge-transfer excited states. In addition, their cellular uptake using breast cancer (MCF-7) and melanoma (SkMel-147 and SkMel-29) cell lines and bioactivity were investigated and their cell-killing mechanism and protein expression were also studied. Preliminary TD-DFT results showed that both compounds exhibited a strong and broad absorption band around 300–400 nm which corresponds to a combination of ILAm2phen and MLCTRe→Am2phen transitions, and a strong contribution of charge transfer transition MLCTRe→et-isonic for fac-[Re(et-isonic)(Am2phen)(CO)3]+ is also observed. In contrast to typical Re(I) polypyridyl complexes, the substitution of Cl with the et-isonic ligand showed a bathochromic shift of the emission maxima, relatively low emission quantum yield and fast lifetime. Photophysical investigation of the fac-[ReCl(et-isonic)2(CO)3] compound provided meaningful information on the excited state manifold of the fac-[Re(L)(Am2phen)(CO)3]0/+ complexes. As shown in the absorption profile, a remarkable inversion of the lowest-lying excited state takes place from the usually observed MLCTRe→Am2phen to the unusual MLCTRe→et-isonic. The lipophilicity of the positive-complex was higher than that of the non-charge compound and the same trend for the activity against cells was observed, in the absence of light. In addition, flow cytometry and Western Blot analyses showed an overexpression of pro-caspase-9, suggesting a caspase proteolytic cascade through an intrinsic-pathway apoptosis mechanism. The photophysical properties of these compounds reported herein provide new fundamental insights into the understanding of substituent groups on polypyridyl ligands which are relevant to practical development.
- This article is part of the themed collection: New Talent: Americas


 Please wait while we load your content...
Please wait while we load your content...