2,5-Bis-trimethylsilyl substituted boroles†
Abstract
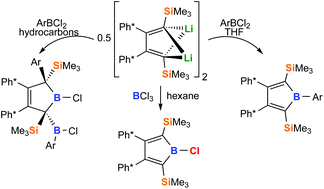
This manuscript includes a comprehensive study of the synthesis and spectroscopic features of 2,5-disilyl boroles. Reacting boron trichloride BCl3 with 2,3-Ph*2-1,4-(SiMe3)2-1,4-dilithiobuta-1,3-diene (Ph* = 3,5-t-Bu2(C6H3)) allowed reliable access to 1-Chloro-2,5-(SiMe3)2-3,4-(Ph*)2-borole in good yields (60%). Unlike 2,3,4,5-tetraphenyl haloboroles, this 2,5-bis-trimethylsilyl substituted chloroborole is thermally stable in solution to up to 130 °C. Metathesis reactions of the chloroborole with metal aryls or of the dilithiobutadiene with arylboron dihalides grant access to 1-Ar-2,5-(SiMe3)2-3,4-(Ph*)2 boroles (Ar = Ph, Mes, Ph*, C6F5). Unlike the generally intensely blue-green 2,3,4,5-tetraaryl boroles, brightly orange/red 2,5-bis-trimethylsilyl substituted boroles reveal blue-shifted π/π*-transitions due to a lack of π-system interaction between borole and 2,5-bound aryls. Light is shed on the synthetic peculiarities for the synthesis of 2,5-disilyl-boroles. While direct treatment of the respective 1,1-dimethyl-stannole with ArBCl2via otherwise well-established B/Sn exchange reactions fails, the selectivity of reactions of 2,3-Ph*2-1,4-(SiMe3)2-1,4-dilithiobuta-1,3-diene with ArBCl2 is solvent dependent and leads to rearranged 3-borolenes in hydrocarbons. Gutmann–Beckett analysis reveal reduced Lewis-acidity of disilylboroles compared to pentaphenyl borole.


 Please wait while we load your content...
Please wait while we load your content...