Inclusion of alkyl nitriles by tetra-armed cyclens with styrylmethyl groups†
Abstract
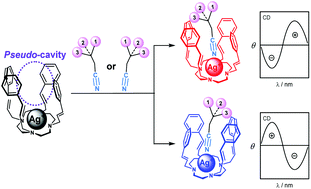
Synthesis of tetra-armed cyclens (2a–2e), with substituted styrylmethyl groups as side-arms, and their Ag+ complexes is reported. The Ag+ complex with a tetra styrylmethyl-armed cyclen (2a) incorporates alkyl nitriles in a pseudo-cavity formed by the four styrylmethyl side-arms. This prompted us to apply this system to the determination of the absolute configurations of chiral nitriles with low [α]D and low circular dichroism (CD) intensity. In the CD spectra of the 2a/Ag+ complex, (S)- and (R)-G1 did not show a specific Cotton effect, while when chiral nitriles were added to the 2a/Ag+ complex, drastic spectral changes were observed. The (S)-G1@2a/Ag+ system exhibited first a negative and then a positive Cotton effect, whereas the (R)-G1@2a/Ag+ system showed the mirror image of the Cotton effect of (S)-G1@2a/Ag+. We have, therefore, demonstrated a new technique for determining the absolute configurations of weak optical rotation molecules using the Ag+ complex with 2a.


 Please wait while we load your content...
Please wait while we load your content...
