The remarkably improved hydrogen storage performance of MgH2 by the synergetic effect of an FeNi/rGO nanocomposite†
Abstract
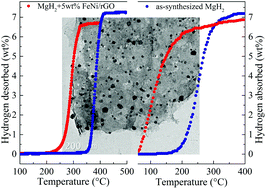
Magnesium hydride (MgH2) has been considered as a promising hydrogen storage material for buildings that are powered by hydrogen energy, but its practical application is hampered by poor kinetics and unstable thermodynamics. Herein, we describe a feasible method for preparing FeNi nanoparticles dispersed on reduced graphene oxide nanosheets (FeNi/rGO), and we confirmed that excellent catalytic effects increased the hydrogen storage performance of MgH2. 5 wt% FeNi/rGO-modified MgH2 began to release hydrogen at 230 °C and liberated 6.5 wt% H2 within 10 min at 300 °C. As for the hydrogenation process, the dehydrogenated sample absorbed 5.4 wt% H2 within 20 min at 125 °C under a hydrogen pressure of 32 bar. More importantly, a hydrogen capacity of 6.9 wt% was maintained after 50 cycles without compromising the kinetics during each cycle. A unique catalytic mechanism promoted synergetic effects between the in situ-formed Mg2Ni/Mg2NiH4, Fe, and rGO that efficiently promoted hydrogen dissociation and diffusion along the Mg/MgH2 interface, anchored the catalyst, and prevented MgH2 from aggregation and growth.


 Please wait while we load your content...
Please wait while we load your content...