Exchange coupled Co(ii) based layered and porous metal–organic frameworks: structural diversity, gas adsorption, and magnetic properties†
Abstract
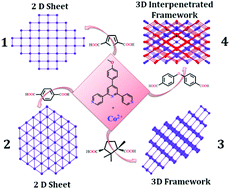
Four new Co(II) based metal–organic frameworks (MOFs) ({[Co3(L)(TDCA)3(DMF)2]n·2nCH3CN}) (1), ({[Co3(L)2(BDCA)3]n·2nCH3CN}) (2), {[Co2(L)2(CA)2]n·4nCH3CN} (3) and {[Co2(L)(OBBA)2]n·3nCH3CN} (4) are synthesized, where L is [4′-(4-methoxyphenyl)-4,2′:6′,4′′-terpyridine], a V-shaped flexible neutral spacer, and the four dicarboxylates are TDCA = thiophene 2,5-dicarboxylic acid, BDCA = benzene 1,4-dicarboxylic acid, CA = (1R,3S)-(+)-camphoric acid and OBBA = 4,4′-oxybisbenzoic acid. Structural analysis reveals that 1 and 2 are two dimensional (2D) layered structures having interesting sql and hxl topologies respectively with trinuclear SBUs (secondary building units). Compound 3 has a 3D structure, whereas 4 has a 2-fold interpenetrated 3D packing structure with a paddlewheel dinuclear SBU and both have pcu topology. Magnetic investigation revealed that 1, 3 and 4 show dominant antiferromagnetic behavior, while 2 shows ferromagnetic interaction at very low temperature. Interestingly 4 shows a sharp decrease in the χMT value from room temperature and this may be because of the direct Co(II)⋯Co(II) interaction. Gas sorption studies reveal that 1, 2 and 3 show surface areas of 11.8 m2 g−1, 8.3 m2 g−1 and 28.5 m2 g−1 respectively and better adsorption behavior for CO2 over CH4, whereas 4 is nonporous in nature due to its 2-fold interpenetrated structure.
- This article is part of the themed collection: Inorganic Porous and Layered Material


 Please wait while we load your content...
Please wait while we load your content...