Effect of positional isomers on the photochromic behaviors of bipyridyltriazolium chloroantimonate hybrids†
Abstract
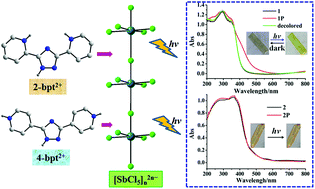
Two chloroantimonate hybrids with isomeric bipyridyltriazoliums and similar packing patterns, {[2-bpt]2[(SbCl5)Cl2]}n (1) and {[4-bpt]2[(SbCl5)Cl2]}n (2) (2-bpt2+ = protonated 3,5-bis(pyridine-2-yl)-1,2,4-triazole, 4-bpt2+ = protonated 3,5-bis(pyridine-4-yl)-1,2,4-triazole), have been designed and synthesized. Distinct intermolecular electronic interactions and photochromic behaviors are attributed to the remarkable modulation of positional isomeric effect on the electron deficiency of the acceptors and donor–acceptor matching relationship. 1 is the first reported photochromic chloroantimonate hybrid.


 Please wait while we load your content...
Please wait while we load your content...