Evaluation of cobalt complexes with tripod ligands for zinc finger targeting†
Abstract
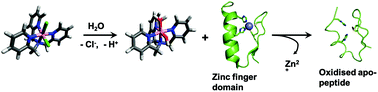
Cobalt complexes have been demonstrated to target zinc fingers, as shown by investigations of Doxovir, the trade name of the [CoIII(acacen)(2-Me-Imz)2]+ drug in clinical trials. Mechanistic studies indicate zinc finger disruption by metal coordination to His residues. Other than Doxovir, a few studies have investigated other ligands and geometries for cobalt complexes for zinc finger targeting. Tripod ligands demonstrated good zinc and cobalt chelation. In this manuscript, we report the ability of CoII and CoIII complexes of tri(2-pyridylmethyl)amine and N,N-di(2-pyridylmethyl)glycinate to disrupt zinc fingers. The results obtained by mass spectrometry and X-ray absorption spectroscopy demonstrate that the complexes were able to remove zinc from the zinc fingers. The product was oxidised apo-peptide. In contrast, the ligands themselves were able to remove zinc, and they did not promote oxidation, resulting in free Cys residues. Cobalt finger adducts were not detected for the complexes with tripod ligands unless they were coordinated to planar ligands such as salen or acacen. Studies of the interactions of cobalt complexes with amino acids demonstrated that tripod ligands promote the cysteine reaction, while the salen ligands promote histidine coordination, demonstrating a different mechanism of action. The results reported here are significant for better understanding and further design of zinc finger targeting compounds.
- This article is part of the themed collection: New Talent: Americas


 Please wait while we load your content...
Please wait while we load your content...