A resorcinarene-based tetrabenzoimidazolylidene complex of rhodium†
Abstract
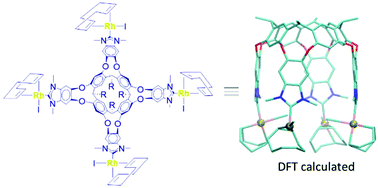
A tetrabenzoimidazolium-resorcinarene cavitand was used for the preparation of a tetra-benzoimidazolylidene of rhodium, which is unprecedented in the field of poly-NHC metal complexes. Both the experimental and computational analyses of the molecule reveal a distorted vase conformation as the most stable one, although several non-interconverting conformational isomers due to the restricted rotation about the Rh–C(carbene) bond coexist in the product. There is a fluxional behaviour involving the vase-kite interconversion. The main interactions between the arms of the cavitand are mostly concentrated on the terminal organometallic fragments attached to NHC, along with those between –CH3 and a N-heterocyclic carbene ring from benzoimidazoles.


 Please wait while we load your content...
Please wait while we load your content...