A study of composition effects on the bandgaps in a series of new alkali metal aluminum/gallium iodates†
Abstract
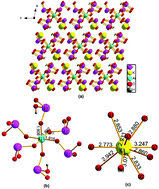
Four new alkali metal aluminum/gallium iodates, namely A2M(IO3)4(H0.5IO3)2 (A = K, Rb and M = Al, Ga), have been successfully synthesized via a hydrothermal reaction and structurally characterized through single-crystal X-ray diffraction analysis. These compounds are isostructural and crystallize in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (no. 2) with one formula unit in each unit cell. The rare half-protonated H0.5IO3 building units were identified, and their structure feature isolated [M(IO3)4(H0.5IO3)2]2− anions, which are separated by alkali metal cations. The optical diffuse reflectance spectroscopy measurements associated with the Tauc analysis showed direct energy gaps, which are in good agreement with the theoretical calculation prediction. Moreover, this study also indicates that the electronic properties of these compounds are mainly determined by the IO3 and H0.5IO3 anionic units, and the alkali metal cations show a remarkable effect on their energy gap values, whereas the impacts of the group 13 elements aluminum and gallium are insignificant.
space group (no. 2) with one formula unit in each unit cell. The rare half-protonated H0.5IO3 building units were identified, and their structure feature isolated [M(IO3)4(H0.5IO3)2]2− anions, which are separated by alkali metal cations. The optical diffuse reflectance spectroscopy measurements associated with the Tauc analysis showed direct energy gaps, which are in good agreement with the theoretical calculation prediction. Moreover, this study also indicates that the electronic properties of these compounds are mainly determined by the IO3 and H0.5IO3 anionic units, and the alkali metal cations show a remarkable effect on their energy gap values, whereas the impacts of the group 13 elements aluminum and gallium are insignificant.


 Please wait while we load your content...
Please wait while we load your content...