Diverse structure and reactivity of pentamethylcyclopentadienyl antimony(iii) cations†
Abstract
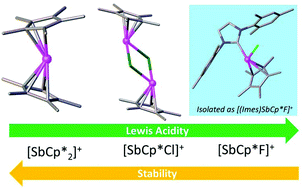
The pentamethylcyclopentadienyl (Cp*) antimony(III) cations [Cp*2Sb][B(C6F5)4], [Cp2*Sb][OTf], [Cp*SbCl][B(C6F5)4] and [Cp*Sb][OTf]2 have been isolated and structurally characterised. [Cp*SbCl]+ forms dimers in the solid state via an intermolecular Sb–Cl interaction. Initial screening shows that [Cp*SbCl][B(C6F5)4] is significantly Lewis acidic and can catalyse the dimerisation of 1,1-diphenylethylene; [Cp2*Sb][B(C6F5)4] exhibits negligible Lewis acidity. Highly unstable [Cp*SbF][B(C6F5)4] could not be isolated, but stabilisation with the IMes ligand allowed isolation of [Cp*SbF(IMes)][B(C6F5)4]. Fluorodechlorination of CH2Cl2 and PhCCl3 was observed in the presence of crude [Cp*SbF][B(C6F5)4] in solution. A computational mechanistic investigation suggests that the latter proceeds via a carbocation intermediate.


 Please wait while we load your content...
Please wait while we load your content...