Selective Th(iv) capture from a new metal–organic framework with O− groups†
Abstract
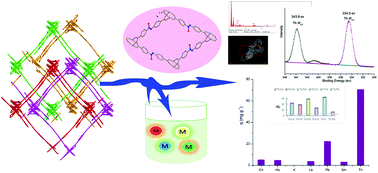
Herein we report a new 4-fold interpenetrated metal–organic framework (MOF) functionalized with O− groups for selective Th(IV) capture, the activated samples 1a exhibited a high adsorption capacity for pure Th(IV) ions (Kd = 3.16 × 105 mL g−1) and the amount of metal ions adsorbed on the adsorbent was 165.61 mg g−1. A high removal efficiency of 99.75% was achieved within 10 min with an initial Th(IV) concentration of 100 mg L−1 and the adsorption data followed the pseudo-second-order model. In addition, the separation coefficient (S) of Th(IV) to metal ions with different valence states such as Th(IV)/La(III), Th(IV)/Sm(III), Th(IV)/Ho(III), Th(IV)/Cd(II) and Th(IV)/K(I) achieved values of 19.66, 26.83, 16.90, 11.26 and 255.79, respectively. Even given the fact that MOFs with O− groups showed high affinity for Pb(II) ions, our adsorption studies for compound 1a revealed a separation coefficient (STh(IV)/Pb(II)) of 4.36. Further, the adsorption of Th(IV) ions to compound 1a was investigated by FT-IR, SEM-EDS and XPS.


 Please wait while we load your content...
Please wait while we load your content...