Aerobic dehydrogenation of amines to nitriles catalyzed by triazolylidene ruthenium complexes with O2 as terminal oxidant†
Abstract
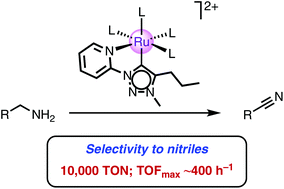
Pyridyl-substituted mesoionic triazolylidene ruthenium cymene complexes catalyze the oxidation of both aromatic and aliphatic amines to nitriles with high activity and selectivity under benign conditions using dioxygen as the terminal oxidant. Modification on the pyridyl moiety of the ligand scaffold has negligible effect on the catalytic performance, while substituents on the triazolylidene directly affect the catalytic fitness of the metal center, leading to distinct catalytic profiles. Pre-dissociation of the cymene ligand and formation of a solvento analogue further enhances the catalytic activity towards nitrile formation. Variation of reaction conditions provided valuable mechanistic insights and resulted in a highly efficient protocol for nitrile formation with maximum turnover numbers around 10 000. The turnover frequency reaches up to 400 h−1, providing one of the fastest catalytic systems known to date for this transformation.


 Please wait while we load your content...
Please wait while we load your content...