Asymmetric hydrogenation of an α-unsaturated carboxylic acid catalyzed by intact chiral transition metal carbonyl clusters – diastereomeric control of enantioselectivity†
Abstract
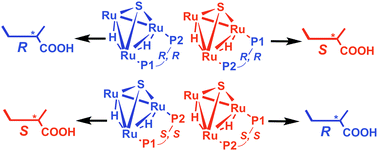
Twenty clusters of the general formula [(μ-H)2Ru3(μ3-S)(CO)7(μ-P–P*)] (P–P* = chiral diphosphine of the ferrocene-based Walphos or Josiphos families) have been synthesised and characterised. The clusters have been tested as catalysts for asymmetric hydrogenation of tiglic acid [trans-2-methyl-2-butenoic acid]. The observed enantioselectivities and conversion rates strongly support catalysis by intact Ru3 clusters. A catalytic mechanism involving an active Ru3 catalyst generated by CO loss from [(μ-H)2Ru3(μ3-S)(CO)7(μ-P–P*)] has been investigated by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...