The solvent and zinc source dual-induced synthesis of a two dimensional zeolitic imidazolate framework with a farfalle-shape and its crystal transformation to zeolitic imidazolate framework-8†
Abstract
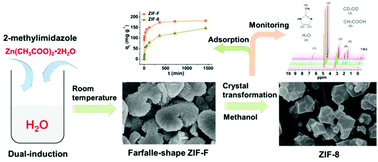
Exploring new zeolitic imidazolate frameworks (ZIFs) with specific topologies and pore structures is important for extending applications and improving performances. In this work, a new farfalle-shaped ZIF with an ordered hierarchical structure (named ZIF-F) was easily built with zinc acetate and 2-methylimidazole (MeIm) in an aqueous system at room temperature. The synthesis mechanism of ZIF-F is a dual-induction interaction of a solvent and zinc source based on the synthesis protocol of ZIF-8. The prepared ZIF-F is a 3–5 μm dispersible particle constructed from numerous nanoplates with the same building units as ZIF-8. ZIF-F has a rich 4 nm inter-particle spacing with a 0.1074 cm3 g−1 total pore volume and exhibits high thermo- and solvent stability. It is worth noting that crystal transformation could occur from ZIF-F to ZIF-8 in methanol via the dissolution-recrystallization route. Regarding the adsorption of Congo red (CR), ZIF-F exhibits better adsorption capacity (182.82 mg g−1) than ZIF-8 (149.25 mg g−1) with 6 times higher adsorption rate than that of ZIF-8 because of the positive effect of its larger pore size and hierarchical structure.


 Please wait while we load your content...
Please wait while we load your content...