Appraisal of calcium ferrites as cathodes for calcium rechargeable batteries: DFT, synthesis, characterization and electrochemistry of Ca4Fe9O17†
Abstract
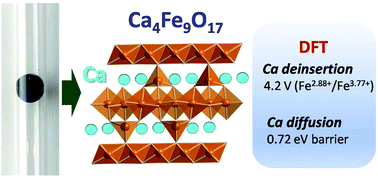
Sustainability combined with high energy density prospects makes Fe-based oxides attractive as cathodes for calcium rechargeable batteries. This work presents a DFT evaluation of the CaFe2+nO4+n (0 < n < 3) family, for which both the average intercalation voltage and the theoretical specific capacity decrease with the increasing n value. The term n = 1/4, Ca4Fe9O17, meets the most appealing characteristics: a calculated average voltage of 4.16 V, a theoretical specific capacity of 230 mA h g−1 and the lowest energy barrier for Ca migration so far predicted for an existing oxide (0.72 eV). To overcome the previously reported synthesis difficulties, we employed a novel synthesis procedure in sealed quartz tubes followed by quenching in water. The XRD and SAED patterns of the prepared Ca4Fe9O17 powder reveal a certain degree of stacking defects along the c axis. Attempts to deinsert Ca ions from Ca4Fe9O17 by chemical means (NO2BF4 in ACN) and in electrochemical Ca cells were unsuccessful, although some hints of oxidation are observed in Li cells with the LP30 electrolyte. The suitability of Ca4Fe9O17 as a Ca cathode is pending further studies utilizing Ca-electrolytes with high anodic stability.


 Please wait while we load your content...
Please wait while we load your content...