Using frustrated Lewis pairs to explore C–F bond activation†
Abstract
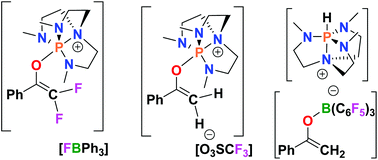
Our interest in C–F bond activation prompted an investigation of the reactions of PhC(O)CF3 with a superbasic proazaphosphatrane (Verkade's base) and a corresponding FLP. The reaction of PhC(O)CF3 with P(MeNCH2CH2)3N in a 2 : 1 ratio generates [FP(NMeCH2CH2)3N][PhC(O)CF2COCF3Ph]. While this salt was not isolable, the anion could be derivatized to allow the isolation of [PhC(O)CF2C(OSiMe2tBu)CF3Ph], 1. To further probe this C–F bond activation, the FLP P(MeNCH2CH2)3N/BPh3 was allowed to react with PhC(O)CF3, which afforded the salt [PhC(CF2)OP(MeNCH2CH2)3N][FBPh3], 2. Insights into the mechanism of the reaction of the proazaphosphatrane with PhC(O)CF3 have emerged from the reactivity of 2 and these have been augmented by DFT computations. Salt 2 could be readily converted to the triflate derivative 3, which was a viable synthon for further C–F bond reactivity. Sequential reaction with Na[HBEt3] afforded the salts [PhC(CF(H))OP(MeNCH2CH2)3N][O3SCF3], 4 and [PhC(CH2)OP(MeNCH2CH2)3N][O3SCF3], 5. Interestingly, the formation of 5 contrasts with the formation of [HP(MeNCH2CH2)3N][PhC(CH2)OB(C6F5)3], 6 observed following the reaction of the FLP P(MeNCH2CH2)3N/B(C6F5)3 with PhC(O)Me. Thus these differing FLP protocols provide avenues to salts containing either an enolate derived cation or anion.
- This article is part of the themed collection: Spotlight Collection: Fluorinated ligands


 Please wait while we load your content...
Please wait while we load your content...