Atomic/molecular layer deposition and electrochemical performance of dilithium 2-aminoterephthalate†
Abstract
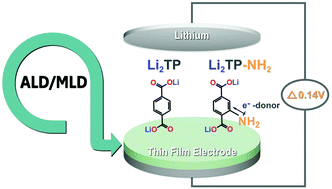
Control of the redox potential of lithium terephthalate Li2TP anode material is demonstrated by functionalizing its terephthalate backbone with an electron-donating amino group; this lowers – as intended – the redox potential of Li2TP by 0.14 V. The two Li-organic electrode materials, Li2TP and Li2TP-NH2, are fabricated as crystalline thin films from gaseous precursors using the atomic/molecular layer deposition (ALD/MLD) technique. The amino-functionalized material possesses a previously unknown crystal structure, addressed here by applying the USPEX evolutionary algorithm for the structure prediction and then LeBail fitting of the experimental XRD pattern based on the predicted structure model. The ALD/MLD fabrication yields in situ lithiated active electrode materials without any conductive additivies or binders and thus allows a straightforward evaluation of their intrinsic electrochemical properties. Comparison between Li2TP and its amino-functionalized derivative reveals inferior capacity retention and rate capability characteristics for the latter, which somewhat counterveils the pros-and-cons balance between the two Li-organic electrode materials. From galvanostatic cycling experiments and post-mortem XRD and SEM analysis, the issue with Li2TP-NH2 is revealed to be in the morphology changes occurring during the discharge/charge cycling.
- This article is part of the themed collection: Spotlight Collection: Atomic and Molecular Layer Deposition


 Please wait while we load your content...
Please wait while we load your content...