Addition reactions and diazomethane capture by the intramolecular P–O–B FLP: tBu2POBcat†
Abstract
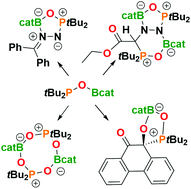
FLPs, R2POBcat (R = tBu 1, Mes 2), are readily derived from the reactions of the corresponding phosphine oxides, nBuLi and ClBcat. Despite the poor Lewis acidity of boron, these species react with PhOH, CO2, CS2, PhNCO, MesCNO, O2, 9,10-phenanthrenedione, and diazomethanes to effect FLP addition reactions affording a series of heterocycles. The reaction of 1 with EtO2CCHN2 gives the bicyclic product, EtO2CCHN2(tBu2POBcat)2. High-level DFT calculations reveal that these cyclization processes proceed via 1,1-addition to the terminal N of diazomethane followed by 1,2-boron-migration affording the five membered rings. The reaction of the EtO2CCHN2 derivative with the second equivalent of FLP 1 is attributed to less steric demands.


 Please wait while we load your content...
Please wait while we load your content...