Functional models of nonheme diiron enzymes: reactivity of the μ-oxo-μ-1,2-peroxo-diiron(iii) intermediate in electrophilic and nucleophilic reactions†‡
Abstract
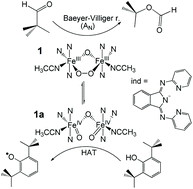
The reactivity of the previously reported peroxo-adduct [FeIII2(μ-O)(μ-1,2-O2)(IndH)2(solv)2]2+ (1) (IndH = 1,3-bis(2-pyridyl-imino)isoindoline) has been investigated in nucleophilic (e.g., deformylation of alkyl and aryl alkyl aldehydes) and electrophilic (e.g. oxidation of phenols) stoichiometric reactions as biomimics of ribonucleotide reductase (RNR-R2) and aldehyde deformylating oxygenase (ADO) enzymes. Based on detailed kinetic and mechanistic studies, we have found further evidence for the ambiphilic behaviour of the peroxo intermediates proposed for diferric oxidoreductase enzymes.


 Please wait while we load your content...
Please wait while we load your content...