A bioinspired thiolate-bridged dinickel complex with a pendant amine: synthesis, structure and electrocatalytic properties†
Abstract
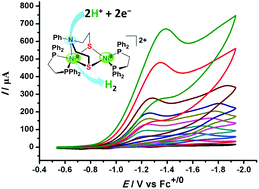
By employing X(CH2CH2S−)2 (X = S, tpdt; X = O, opdt; X = NPh, npdt) as bridging ligands, four thiolate-bridged dinickel complexes supported by phosphine ligands, [(dppe)Ni(μ-13κSSS′:22κSS-tpdt)Ni(dppe)][PF6]2 (1[PF6]2, dppe = Ph2P(CH2)2PPh2), [(dppe)Ni(μ-13κSSN:22κSS-npdt)Ni(dppe)][PF6]2 (2[PF6]2) and [(dppe)Ni(t-Cl)(μ-13κSSX:22κSS-C4H8S2X)Ni(dppe)][PF6] (3[PF6], X = S; 4[PF6], X = O) were facilely obtained by the salt metathesis reaction. These four thiolate-bridged dinickel complexes have all been fully characterized by spectroscopic methods and X-ray crystallography. In 2[PF6]2, elongation of the Ni–N bond distance, possibly caused by steric hindrance, indicates that the pendant nitrogen group shuttles between the two nickel centers in solution, which is evidenced by 31P{1H} NMR spectroscopic results. Furthermore, these thiolate-bridged dinickel complexes have all been proved to be electrocatalysts for proton reduction to hydrogen. Notably, complex 2[PF6]2 featuring a pendant amine group in the secondary coordination sphere exhibits the best catalytic activity at a relatively low overpotential.


 Please wait while we load your content...
Please wait while we load your content...