A novel self-assembled Na{Cu12Zn4} multifunctional material: first report of a discrete coordination compound for detection of Ca2+ ions and selective adsorption of cationic dyes in water†
Abstract
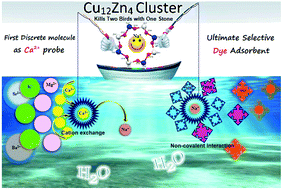
A novel metal directed discrete self-assembled compound, Na2O[Cu12Zn4(μ-OH)8(H4btp)8](ClO4)8·26H2O (1), was synthesized employing a flexible aminoalcohol ligand, 2,2′-(propane-1,3-diyldiimino)bis-[2-(hydroxylmethyl)propane-1,3-diol] (H6btp), and mixed metal ions (CuII and ZnII) in order to explore it as a multifunctional material. The molecular cluster is characterized by spectral and analytical techniques, single crystal X-ray diffraction and magnetic studies. Crystallography reveals the presence of eight peripheral CuII centers in octahedral environments, while four interior CuII and ZnII centers are present in tetrahedral systems. The ligand is coordinated in the dianionic form (H4btp2−) with copper and zinc metal ions through various bridging modes. Magnetic data suggest the presence of strong antiferromagnetic coupling between the twelve Cu(II) centers (C = 4.31 cm3 K mol−1 and θ = −120.44 K). Interestingly, the eight ligands present in the system have eight free hydroxyl groups at the periphery of the cluster, which could be employed in molecular recognition for the analytes under investigation. One of the sodium cations of the lattice sodium oxide [Na2O or Na+(Na+O2−)] is encapsulated in the {Cu12Zn4} cavity and held through electrostatic interactions with the oxygen atoms, forming a Na{Cu12Zn4} core. Taking advantage of this entrapped cation, we have used the present assembly in the sensing of various s-block cations. Interestingly, the cluster shows a sensitive and selective sensing ability towards Ca2+ ions through cation exchange. The selectivity towards Ca2+ can be rationalized in terms of the identical size of the sodium and calcium ions and the HSAB principle. Hard sodium is exchanged with the hard Ca2+ ion, which effectively interacts with the hard oxygen donor, giving rise to a stable hard–hard interaction. Moreover the Na{Cu12Zn4} cluster shows excellent selectivity towards the adsorption of cationic dyes, specifically methylene blue (MB), via non-covalent interactions. An abundance of free O–H groups in the cluster gives rise to the existence of O–H⋯π interactions with the dye for enhanced adsorption. Thus, the present report is the first example of any heterometallic coordination compound (in contrast to complex systems like polymeric frameworks/nanocomposites) with discriminating sensing of Ca2+ ions with ultra-low detection limit (4 nM) and with high efficiency in the selective adsorption and separation of methylene blue.


 Please wait while we load your content...
Please wait while we load your content...