Proton-conducting layered structures based on transition metal oxo-clusters supported by Sb(iii) tartrate scaffolds†
Abstract
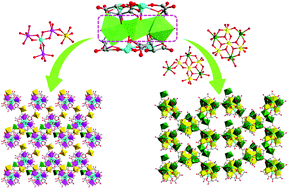
Two transition metal–antimony oxo-cluster based compounds, H5{MCd(H2O)6[M(H2O)3Co3SbVSbIII6(μ3-O)8(L-tta)6]}·7H2O (1) (M = Cd0.5 + Co0.5) and H3K5(H2O)11{Cd(H2O)4[Cd(H2O)Fe4Cd2Sb6(μ4-O)5(μ3-O)3(L-tta)6][Cd(H2O)2Fe4Cd2Sb6(μ4-O)4(μ3-O)4(L-tta)6][Cd(H2O)2Fe4Cd2Sb6(μ4-O)4(μ3-O)4(L-tta)6Cd(H2O)5]}·17H2O (2) (L-H4tta = L-tartaric acid) were hydrothermally synthesized and characterized. Compound 1 features a [MCo3SbVSbIII6(μ3-O)8(L-tta)6(H2O)3]9− cluster, while compound 2 contains three types of clusters, namely, [Cd(H2O)Fe4Cd2Sb6(μ4-O)5(μ3-O)3(L-tta)6]4−, [Cd(H2O)2Fe4Cd2Sb6(μ4-O)4(μ3-O)4(L-tta)6]4− and [Cd(H2O)2Fe4Cd2Sb6(μ4-O)4(μ3-O)4(L-tta)6Cd(H2O)5]2−. All the clusters are of sandwich-type with {Sb3(μ3-O)(L-tta)} scaffolds on the top and bottom. The Cd (and M in 1) ions interconnect the clusters into layered structures in both compounds. To the best of our knowledge, this is the first report of transition metal–antimony oxo-clusters that simultaneously contain the first-row and second-raw transition metal ions, and compound 1 represents the first example of such type of clusters that contain Sb(V). The two compounds exhibit proton conductivity with the values of 2.43 × 10−3 and 2.95 × 10−3 S cm−1 at 85 °C under 98% relative humidity, respectively.
- This article is part of the themed collection: Inorganic Porous and Layered Material


 Please wait while we load your content...
Please wait while we load your content...