A novel water-resistant and thermally stable black lead halide perovskite, phenyl viologen lead iodide C22H18N2(PbI3)2†
Abstract
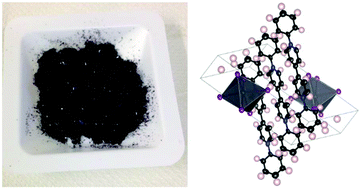
A novel black organoammonium iodoplumbate semiconductor, namely phenyl viologen lead iodide C22H18N2(PbI3)2 (PhVPI), was successfully synthesized and characterized. This material showed physical and chemical properties suitable for photovoltaic applications. Indeed, low direct allowed band gap energy (Eg = 1.32 eV) and high thermal stability (up to at least 300 °C) compared to methylammonium lead iodide CH3NH3PbI3 (MAPI, Eg = 1.5 eV) render PhVPI potentially attractive for solar cell fabrication. The compound was extensively characterized by means of X-ray diffraction (performed on both powder and single crystals), UV-Vis diffuse reflectance spectroscopy (UV-Vis DRS), UV-photoelectron spectroscopy (UPS), FT-IR spectroscopy, TG-DTA, and CHNS analysis. Reactivity towards water was monitored through X-ray powder diffraction carried out after prolonged immersion of the material in water at room temperature. Unlike its methyl ammonium counterpart, PhVPI proved to be unaffected by water exposure. The lack of reactivity towards water is to be attributed to the quaternary nature of the nitrogen atoms of the phenyl viologen units that prevents the formation of acid–base equilibria when in contact with water. On the other hand, PhVPI's thermal stability was evaluated by temperature-controlled powder XRD measurements following an hour-long isothermal treatment at 250 and 300 °C. In both cases no signs of decomposition could be detected. However, the compound melted incongruently at 332 °C producing, upon cooling, a mostly amorphous material. PhVPI was found to be slightly soluble in DMF (∼5 mM) and highly soluble in DMSO. Nevertheless, its solubility in DMF can be dramatically increased by adding an equimolar amount of DMSO. Therefore, phenyl viologen lead iodide can be amenable for the fabrication of solar devices by spin coating as actually done for MAPI-based cells. The crystal structure, determined by means of single crystal X-ray diffraction using synchrotron radiation, turned out to be triclinic and consequently differs from the prototypal perovskite structure. In fact, it comprises infinite double chains of corner-sharing PbI6 octahedra along the a-axis direction with phenyl viologen cations positioned between the columns. Finally, the present determination of PhVPI's electronic band structure achieved through UPS and UV-Vis DRS is instrumental in using the material for solar cells.


 Please wait while we load your content...
Please wait while we load your content...