Morphology control of metal-modified zirconium phosphate support structures for the oxygen evolution reaction†
Abstract
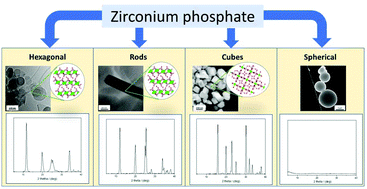
The electrochemical oxygen evolution reaction (OER) is the half-cell reaction for many clean-energy production technologies, including water electrolyzers and metal–air batteries. However, its sluggish kinetics hinders the performance of those technologies, impeding them from broader implementation. Recently, we reported the use of zirconium phosphate (ZrP) as a support for transition metal catalysts for the oxygen evolution reaction (OER). These catalysts achieve promising overpotentials with high mass activities. Herein, we synthesize ZrP structures with controlled morphology: hexagonal platelets, rods, cubes, and spheres, and subsequently modify them with Co(II) and Ni(II) cations to assess their electrochemcial OER behavior. Through inductively coupled plasma mass-spectrometry measurements, the maximum ion exchange capacity is found to vary based on the morphology of the ZrP structure and cation selection. Trends in geometric current density and mass activity as a function of cation selection are discussed. We find that the loading and coverage of cobalt and nickel species on the ZrP supports are key factors that control OER performance.
- This article is part of the themed collection: Inorganic Porous and Layered Material


 Please wait while we load your content...
Please wait while we load your content...