Exploring new hydrated delta type vanadium oxides for lithium intercalation†
Abstract
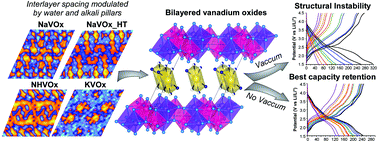
Three hydrated double layered vanadium oxides, namely Na0.35V2O5·0.8(H2O), K0.36(H3O)0.15V2O5 and (NH4)0.37V2O5·0.15(H2O), were obtained by using mild hydrothermal conditions. Their delta type structural frameworks were solved by high-resolution synchrotron X-ray powder diffraction and the interlayer spacings were interpreted from difference Fourier maps. The inter-slab distances are modulated by the water content and the special arrangements of the alkali and ammonium cations. The XPS measurements denote mixed valence systems with high contents of V4+ ions up to 40%. The monitoring of the V4+ EPR signal over time suggests a reduction of the electronic delocalization on account of the partial oxidation to V5+. The electrochemical performance of the active phases is strongly conditioned by the vacuum-drying process of the electrodes, showing better capacity retention when vacuum is not applied. In situ X-ray diffraction shows a structural mechanism of contraction/expansion of the bilayers upon lithium insertion/extraction where the alkali ions behave as structural stabilizers. Galvanostatic cycling at very low current density implies migration of the alkali “pillars” triggering the collapse of the structure.
- This article is part of the themed collection: Inorganic Porous and Layered Material


 Please wait while we load your content...
Please wait while we load your content...