Insights from semi-oriented EPR spectroscopy studies into the interaction of lytic polysaccharide monooxygenases with cellulose†
Abstract
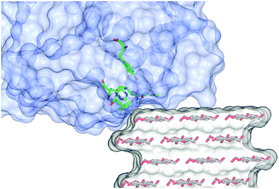
Probing the detailed interaction between lytic polysaccharide monooxygenases (LPMOs) and their polysaccharide substrates is key to revealing further insights into the mechanism of action of this class of enzymes on recalcitrant biomass. This investigation is somewhat hindered, however, by the insoluble nature of the substrates, which precludes the use of most optical spectroscopic techniques. Herein, we report a new semi-oriented EPR method which evaluates directly the binding of cellulose-active LPMOs to crystalline cellulose. We make use of the intrinsic order of cellulose fibres in Apium graveolens (celery) to orient the LPMO with respect to the magnetic field of an EPR spectrometer. The subsequent angle-dependent changes observed in the EPR spectra can then be related to the orientation of the g matrix principal directions with respect to the magnetic field of the spectrometer and, hence, to the binding of the enzyme onto the cellulose fibres. This method, which does not require specific modification of standard CW-EPR equipment, can be used as a general procedure to investigate LPMO–cellulose interactions.
- This article is part of the themed collections: Celebrating our 2020 Prize and Award winners and Breaking bonds over many timescales: in celebration of Robin Perutz’s 70th birthday


 Please wait while we load your content...
Please wait while we load your content...