Tuning a modular system – synthesis and characterisation of a boron-rich s-triazine-based carboxylic acid and amine bearing a galactopyranosyl moiety†
Abstract
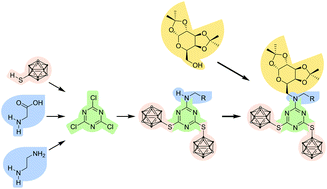
Introduction of a bis(isopropylidene)-protected galactopyranosyl moiety in s-triazine-based boron-rich carboxylic acids and amines results in soluble and suitable coupling partners for tumour-selective biomolecules with applications as selective agents for boron neutron capture therapy (BNCT). Bearing either a carboxylic acid or primary amine as a functional group, these compounds are highly versatile and thus largely extend the possible coupling strategies with suitable biomolecules. Modification of the gastrin-releasing peptide receptor (GRPR) selective agonist [D-Phe6, β-Ala11, Ala13, Nle14]Bn(6–14) with the carboxylic acid derivative yielded a bioconjugate with an optimal receptor activation and internalisation profile. This demonstrates the great potential of this approach for the development of novel boron delivery agents.


 Please wait while we load your content...
Please wait while we load your content...