A cobalt hydroxide nanosheet-mediated synthesis of core–shell-type Mn0.005Co2.995O4 spinel nanocubes as efficient oxygen electrocatalysts†
Abstract
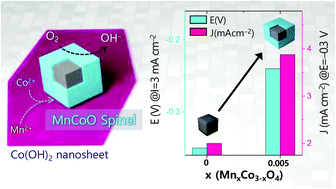
We developed a topotatic growth method involving an exfoliated cobalt hydroxide nanosheet, which allows water-based mild reaction conditions (90 °C) for the formation of the homogeneous cubic structure of MnxCo3−xO4 spinel oxides with Mn(II)/Co(II) salts. The size of the nanocubes increased as the Mn content increased, e.g., 13 nm (x = 0.0), 23 nm (x = 0.005), 50 nm (x = 0.05), and 140 nm (x = 1.0). The incorporation of Mn into Co3O4 dramatically increased the ORR performance because the catalytically active Mn cations exclusively substitute the less active Co2+ in the MnxCo3−xO4 structure. We effectively reduced the Mn content in the spinel Co3O4 structure to a value of 0.005, representing the Mn0.005Co2.995O4 spinel nanocubes that unexpectedly exhibited the best ORR activity among the samples. In addition, the XPS and ICP characterizations suggest an Mn-rich shell/Co-rich core for the MnxCo3−xO4 nanocubes.


 Please wait while we load your content...
Please wait while we load your content...