Solid state characterization of oxidized actinides co-crystallized with uranyl nitrate hexahydrate†
Abstract
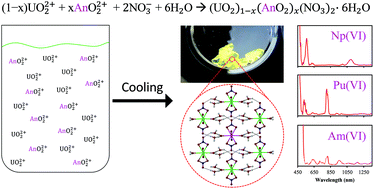
Characterization of the penta- and hexavalent dioxo cations of NpO2+, NpO22+, PuO22+, and AmO22+ has been carried out by diffuse reflectance UV-Vis-NIR spectroscopy, with the first observations of NpO2+, NpO22+, and AmO22+ in the solid state. Absorbance measurements confirmed the presence of the higher actinides of Np, Pu, and Am, with shifts in their absorbance bands indicating the formation of the dinitrate species in the crystalline phase. The oxidized actinides were prepared in the solid state by co-crystallization with UO2(NO3)2·6H2O by a simple reduction in temperature. The hexavalent species were all co-crystallized in near proportion to UO22+ and the pentavalent species was co-crystallized in a slightly less efficient manner, roughly 83% of that of UO22+.


 Please wait while we load your content...
Please wait while we load your content...