Synthesis and antiproliferative activity of benzimidazole-based, trinuclear neutral cyclometallated and cationic, N^N-chelated ruthenium(ii) complexes†
Abstract
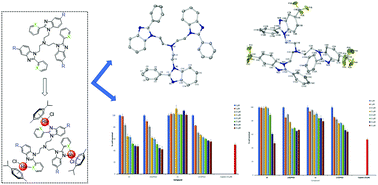
A series of 2-phenyl and 2-pyridyl tris-benzimidazole ligands was reacted with the [Ru(p-cymene)Cl2]2 dimer to yield the corresponding neutral cyclometallated and cationic organoruthenium(II) complexes. All of the synthesized compounds were characterized using an array of spectroscopic and analytical techniques, including 1H, 13C nuclear magnetic resonance (NMR), infrared spectroscopy and mass spectrometry. The trinuclear compounds were screened for their cytotoxicy against the MCF-7 breast cancer cell line and the triple negative MDA-MB-231 breast cancer cell line at concentrations of 10 and 20 μM. Overall, the 2-pyridyl ligands 10 and 11, and their corresponding trinuclear complexes [16][PF6]3 and [17][PF6]3, show the most promising activity as these compounds significantly reduce the percentage cell survival of MCF-7 and MDA-MB-231 breast cancer cell lines at the aforementioned fixed concentrations. It was observed that 10 and [16][PF6]3 show potency greater than that of cisplatin against the MCF-7 breast cancer cell line, and [17][PF6]3 shows potency comparable to that of cisplatin against the MCF-7 cell line. Additionally, the synthesized compounds were observed to have relatively low cytotoxicty towards MCF-12A breast epithelial cells and relatively higher selectivity towards MCF-7 breast cancer cells compared to cisplatin.


 Please wait while we load your content...
Please wait while we load your content...