Use of lithium aryloxides as promoters for preparation of α-hydroxy acid esters†
Abstract
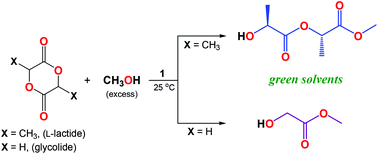
In this work, a hexanuclear lithium compound, [Li6(MesalO)6] (1), supported by a chelating ligand, namely methyl salicylato (MesalOH), was used as a precursor for preparation of the monomeric lithium aryloxides [Li(MesalO)(MesalOH)] (2) and [Li(MesalO)(MeOH)2] (3) via reactions with MesalOH or MeOH. These aryloxides were characterized by single-crystal X-ray diffraction, and spectroscopic and other analytical methods. The diffusion-ordered 1H NMR measurements revealed the retention of solid-state structures of 1 and 2 in THF-d8 solution. Experimental data obtained for 3 showed its decomposition into compound 1 and free MeOH. Compound 1 generated from 3 was also used as a catalyst for the alcoholysis of L-lactide (L-LA) and glycolide (GA) for the preparation of α-hydroxy acid esters. We established that during methanolysis in the presence of 1, L-LA was selectively transformed into methyl (S,S)-O-lactyllactate (MeL2), and GA was converted to methyl glycolate (MeG1) and oligoglycolate esters MeGn (n = 2, 3, and 4).


 Please wait while we load your content...
Please wait while we load your content...