Influence of catalyst nuclearity on copper-catalyzed aerobic alcohol oxidation†
Abstract
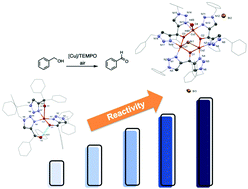
Reactions of CuX with the bis(triazolyl) ligand Hbtm [bis(1-benzyl-1H-1,2,3-triazol-4-yl)phenylmethanol] in CH2Cl2 afforded trinuclear copper(II) complexes with a core structure (μ-X)Cu3(μ-κ3-N,O,N-btm)3(L)2+ [X = Cl, L = CH3OH (1); X = Br, L = H2O (2)], while a similar reaction of [Cu(CH3CN)4](PF6) with the mono(triazolyl) ligand HPhtm [(1-benzyl-1H-1,2,3-triazol-4-yl)diphenylmethanol] resulted in the mononuclear complex [Cu(κ2-N,O-Phtm)(κ2-N,O-HPhtm)(κ1-N-HPhtm)][PF6] (3). The structural characterization of these complexes was made by single-crystal X-ray crystallography in combination with elemental and ESI mass analyses. Catalytic studies toward aerobic oxidation of benzyl alcohol to benzaldehyde revealed that the trinuclear 1 and 2 exhibited higher activities than the mononuclear 3 in both CH3CN and EtOH/H2O solvent systems.


 Please wait while we load your content...
Please wait while we load your content...