Dicerium letterbox-shaped tetraphenolates: f-block complexes designed for two-electron chemistry†
Abstract
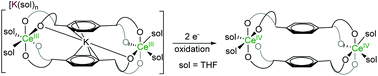
Rare examples of molecular, dinuclear CeIII and PrIII complexes with robust Ln-coordination are accessible by use of the tetraphenolate pTP as a supporting, chelating O-donor ligand platform, pTP = [{2-(OC6H2R2-2,4)2CH}-C6H4-1,4]4− that favours the higher formal oxidation states accessible to rare earths. Two classes of complexes have been made from the platforms; one metallacyclic 2 + 2 [Ln2(pTP)2] framework with a rigid, letterbox-shaped geometry and [Ln(aryloxide)4] core, and one more flexible [(LnX)2(pTP)] with one rare earth ion at either end of the platform. The LnIII letterbox complexes have two K+ counter-cations, one of which sits inside the letterbox, binding the two central arenes of the platform sufficiently strongly that it cannot be displaced by solvent molecules (THF and pyridine) or crown ethers. Oxidation of the CeIII lettterboxes is facile and forms the unusual neutral molecular (CeIV)2 letterbox in which the CeIV reduction potential is −1.83 V vs. Fc/Fc+. The electronic structure of the Ce(III/IV) complexes was investigated using HERFD-XAS (high energy resolution fluorescence detection X-ray absorption spectroscopy).
- This article is part of the themed collection: Breaking bonds over many timescales: in celebration of Robin Perutz’s 70th birthday


 Please wait while we load your content...
Please wait while we load your content...