A highly sensitive and selective colorimetric probe based on a cycloruthenated complex: an Hg2+-promoted switch of thiophene coordination†
Abstract
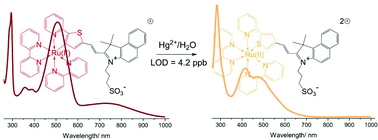
A cyclometalated ruthenium complex [Ru(pthb)(bpy)2]+ (1, bpy = 2,2′-bipyridine, Hpthb = 3,3-dimethyl-2-(5-pyridylthiophen-2-yl)vinyl-benzo[e]indolium-1-propylsulfonate) could be converted from a C-coordinated structure to non-metallated species with N,S-bonded Hpthb upon treatment with mercury(II) ions in water. Strikingly, the switch in the coordination mode resulted in a great absorption change along with a change in the solution color of 1 from dark red to light yellow. Therefore, 1 can be used as a colorimetric probe to detect mercury(II) ions by the naked eye. Although the emission was not observed for 1 in water, it still demonstrated an appreciably low detection limit of 21 nM by using UV–Vis absorption spectroscopy, which was comparable with those of some probes determined by ratiometric fluorescence spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...