Investigating the effect of positional isomerism on the assembly of zirconium phosphonates based on tritopic linkers†
Abstract
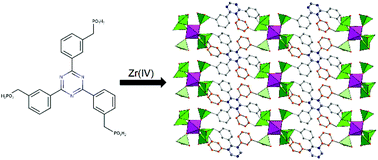
We report on the use of a novel tritopic phosphonic linker, 2,4,6-tris[3-(phosphonomethyl)phenyl]-1,3,5-triazine, for the synthesis of a layered zirconium phosphonate, named UPG-2. Comparison with the structure of the permanently porous UPG-1, based on the related linker 2,4,6-tris[4-(phosphonomethyl)phenyl]-1,3,5-triazine, reveals that positional isomerism disrupts the porous architecture in UPG-2 by preventing the formation of infinitely extended chains connected through Zr–O–P–O–Zr bonds. The presence of free, acidic P–OH groups and an extended network of hydrogen bonds makes UPG-2 a good proton conductor, reaching values as high as 5.7 × 10−4 S cm−1.
- This article is part of the themed collection: Inorganic Porous and Layered Material


 Please wait while we load your content...
Please wait while we load your content...