Mechanistic insights of selective syngas conversion over Zn grafted on ZSM-5 zeolite†
Abstract
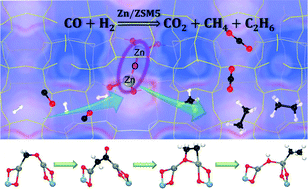
Metal-exchanged zeolites have attracted broad interest in alkane activation, NH3 selective catalytic reduction (NH3-SCR) reactions etc. Very recently, Chen et al. (Angew. Chem. Int. Ed. 2020, 59, 6529–6534) reported that Zn2+-ion exchanged aluminosilicate zeolites catalyze syngas conversion efficiently toward ethane with a high selectivity. Although the main active site of the catalyst has been proposed to be [Zn–O–Zn]2+ by solid state NMR experiments, the detailed reaction mechanism remains to be elucidated. Based on the intermediates detected experimentally, theoretical calculations herein propose a reaction pathway for ethane formation along with the intermediates [Zn–OCH2–Zn]2+ → [Zn–CH2CO–Zn]2+ → [Zn–OCH2CH2–Zn]2+ → [Zn–OH–Zn–C2H5]2+ grafted on a ZSM-5 zeolite. Combined with the kinetic analysis of the reaction pathways and the Gibbs free energy surface of alkane formation, the overall syngas conversion to ethane is nicely consistent with the experimental observations. On the basis of the evolution of the natural orbitals along the reaction coordinates, ZSM-5 not only provides an environment on which to graft the highly dispersed Zn species as [Zn–O–Zn]2+ but also decreases the barriers by efficient charge transfer. The in-depth understanding gained in this work may provide important guidance for the more rational design and optimization of catalysts for syngas conversion.


 Please wait while we load your content...
Please wait while we load your content...