Solvent-free ketalization of polyols over germanosilicate zeolites: the role of the nature and strength of acid sites†
Abstract
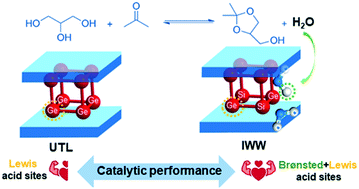
Isomorphic substitution of silicon for germanium affords germanosilicate zeolites with weak acid centers capable of catalyzing key reactions such as Baeyer–Villiger oxidation of ketones and etherification of levulinic acid. Herein, we show for the first time that UTL (Si/Ge = 4.2) and IWW (Si/Ge = 7.2) germanosilicate zeolites are active and selective catalysts of polyol (e.g., ethylene glycol, glycerol and 1,4 butanediol) ketalization to dioxolanes. Large-pore IWW outperformed the extra-large-pore UTL zeolite in the ketalization of polyols, thus indicating diffusion limitations in bulky platelet-like UTL crystals. FTIR spectroscopy of adsorbed pyridine revealed the Lewis acidity of the UTL zeolite, whereas the more active IWW catalyst was characterized by water-induced Brønsted acidity. Increasing the activation temperature (200–450 °C) reduced the concentration of Brønsted acid centers in the IWW germanosilicate (i.e., 0.16; 0.07 and 0.05 mmol g−1 for Tact = 200, 300 and 450 °C, respectively) but increased the number of Lewis acid sites in both zeolites. Under optimized reaction conditions (e.g., acetone/glycerol = 25, Tact = 300 °C), almost total transformation of glycerol into solketal was achieved within 3 h of reaction time over the IWW zeolite at room temperature (>99% yield of the target product). The results from the present study clearly show that weak acid centers of germanosilicate zeolites can serve as active sites in ketalization reactions.


 Please wait while we load your content...
Please wait while we load your content...