Synthesis of Co-doped MnO2 catalysts with the assistance of PVP for low-temperature SCR†
Abstract
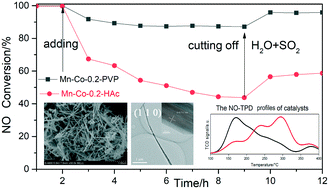
MnO2–CoOX catalysts with different Mn/Co ratios were prepared using polyvinyl pyrrolidone (PVP) as an assistant to control the morphology and exposed crystal faces of composite oxides. The physicochemical properties of the catalysts were systematically characterized and the catalytic activity of NH3-SCR and resistance to SO2/H2O were investigated. It was found that the NO conversion over Mn–Co-0.2-PVP was apparently higher compared with that over Mn–Co-X-PVP (X = 0.9, 0.8, 0.6, and 0.4). This can be interpreted in terms of exposed crystal surface (110) and enhanced adsorption of NH3/NO, and better redox performance resulting from higher Co3+/Co2+ ratio. Furthermore, different PVP and HAc additives have different H2O and SO2 resistance. Mn–Co-0.2-PVP showed good resistance to H2O and SO2 poisoning owing to having more low-temperature (∼200 °C) NO adsorption sites, which are beneficial to promote the de-NOX reaction and less affected by SO2.


 Please wait while we load your content...
Please wait while we load your content...