Direct amide formation in a continuous-flow system mediated by carbon disulfide†
Abstract
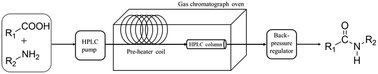
Amide bonds are ubiquitous in nature. They can be found in proteins, peptides, alkaloids, etc. and they are used in various synthetic drugs too. Amide bonds are mainly made by the use of (i) hazardous carboxylic acid derivatives or (ii) expensive coupling agents. Both ways make the synthetic technology less atom economic. We report a direct flow-based synthesis of amides. The developed approach is prominently simple and various aliphatic and aromatic amides were synthetized with excellent yields. The reaction in itself is carried out in acetonitrile, which is considered as a less problematic dipolar aprotic solvent. The used coupling agent, carbon disulfide, is widely available and has a low price. The utilized heterogeneous Lewis acid, alumina, is a sustainable material and it can be utilized multiple times. The technology is considerably robust and shows excellent reusability and easy scale-up is carried out without the need of any intensive purification protocols.


 Please wait while we load your content...
Please wait while we load your content...