Room temperature and atmospheric pressure aqueous partial oxidation of ethane to oxygenates over AuPd catalysts†
Abstract
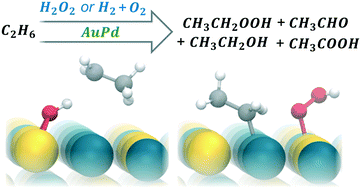
New modes of chemical manufacturing based on small-scale, distributed facilities have been proposed to supplement many existing production operations in the chemical industry, including the synthesis of value-added products from light alkanes. Motivated by this prospect, herein the aqueous partial oxidation of ethane over unsupported AuPd nanoparticle catalysts is investigated, with emphasis on outcomes for reactions occurring at 21 °C and 1 bar ethane. When H2O2 is used as an oxidant, the system generates numerous C2 oxygenates, including ethyl hydroperoxide/ethanol, acetaldehyde, and acetic acid. Ethyl hydroperoxide is found to be the primary product resulting from the direct oxidation of ethane: it is produced with 100% selectivity in batch reactions with short durations and with low initial H2O2 concentrations. At longer times or in more oxidizing conditions, deeper product oxidations expectedly occur. In batch experiments, the maximum observed yield of oxygenates is 7707 μmol gAuPd−1 h−1. Product distributions differ when H2O2 is replaced by H2 and O2 in the headspace. Additionally, to simulate a scenario wherein H2O2 is produced on-site and to study ethane oxidation in steady, low H2O2 concentrations over 50 h, a semi-batch configuration facilitating continuous injection of dilute H2O2 was implemented. These efforts showed that H2O2 can serve as an oxygenate-selective oxidant of ethane when its concentration is kept low during reaction. These and other experimental results, as well as initial computational results using density functional theory, suggest that paths forward for aqueous ethane conversion exist, and systems should be engineered to emphasize product stabilization.


 Please wait while we load your content...
Please wait while we load your content...