Fundamental roles of ZnO and ZrO2 in the conversion of ethanol to 1,3-butadiene over ZnO–ZrO2/SiO2
Abstract
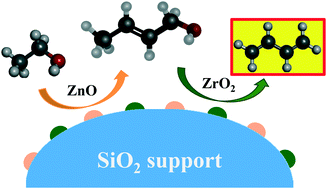
The ZnO–ZrO2/SiO2 catalyst is very active and selective for the conversion of ethanol to 1,3-butadiene (ETB). By varying the method of catalyst preparation we found that there is no synergetic interaction between ZnO and ZrO2 functions; that is, they act as independent catalysts. Estimations through density functional theory calculations showed that the rate-determining step of the ETB reaction is dehydration of crotyl alcohol over ZnO, while the rate-determining step with ZrO2 is ethanol dehydrogenation. The ZnO and ZrO2 sites in the ZnO–ZrO2/SiO2 catalyst act in tandem to catalyze different steps of the ETB reaction.


 Please wait while we load your content...
Please wait while we load your content...