d-Phenylglycine aminotransferase (d-PhgAT) – substrate scope and structural insights of a stereo-inverting biocatalyst used in the preparation of aromatic amino acids†‡
Abstract
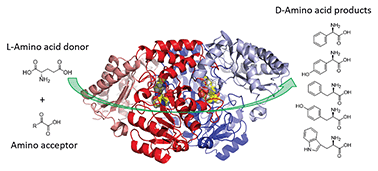
Enantiopure amines are key building blocks in the synthesis of many pharmaceuticals, so a route to their production is a current goal for biocatalysis. The stereo-inverting D-phenylglycine aminotransferase (D-PhgAT), isolated from Pseudomonas stutzeri ST-201, catalyses the reversible transamination from L-glutamic acid to benzoylformate, yielding α-ketoglutarate and D-phenylglycine (D-Phg). Detailed kinetic analysis revealed a range of amine donor and acceptor substrates that allowed the synthesis of enantiopure aromatic D-amino acids at a preparative scale. We also determined the first X-ray crystal structure of D-PhgAT with its bound pyridoxal 5′-phosphate (PLP) cofactor at 2.25 Å resolution. A combination of structural analysis and site-directed mutagenesis of this class III aminotransferase revealed key residues that are potentially involved in the dual substrate recognition, as well as controlling the stereo-inverting behaviour of D-PhgAT. Two arginine residues (Arg34 and Arg407) are involved in substrate recognition within P and O binding pockets respectively. These studies lay the foundation for further enzyme engineering and promote D-PhgAT as a useful biocatalyst for the sustainable production of high value, aromatic D-amino acids.


 Please wait while we load your content...
Please wait while we load your content...