Deep insight into the catalytic removal mechanism of a multi-active center catalyst for chlorobenzene: an experiment and density functional theory study†
Abstract
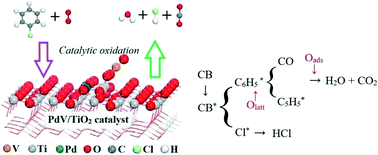
Catalytic oxidation is a promising dioxin purification removal technique due to high efficiency and low consumption, as well as no secondary pollution. In this work, a multi-active center catalytic system was prepared, and the catalytic removal of chlorobenzene (CB) was investigated. The results showed excellent CB conversion and CO2 selectivity, especially the Pd0.12V4/TiO2 catalyst with the lowest T90 (256.6 °C) and activation energy (Ea) (8.29 kJ mol−1). XRD, TEM, XPS, H2-TPR, in situ DRIFT, and DFT analysis were carried out to examine the CB catalytic removal mechanism. The obtained data revealed that all components (PdOx, VOx and TiO2) of the catalyst play an indispensable role in CB oxidation removal, and the redox cycle (2V4+ (Ti3+) + Pd2+ ↔ 2V5+ (Ti4+) + Pd0) was attributed to its superior CB catalytic performance. Additionally, a possible catalytic removal transformation mechanism of CB by the Pd0.12V4/TiO2 catalyst was proposed, which was consistent with the experimental and in situ DRIFT spectroscopy results.


 Please wait while we load your content...
Please wait while we load your content...