One-pot efficient biosynthesis of (3R)-acetoin from pyruvate by a two-enzyme cascade†
Abstract
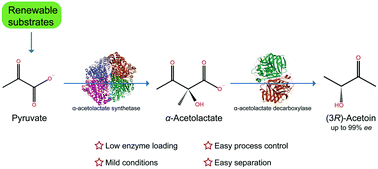
Acetoin, especially its enantiomers (3R)- and (3S)-acetoin, is a high-value added bio-based platform chemical with wide applications in the food, cosmetic, agricultural and chemical industries, which consequently gains great attention for its efficient biosynthesis. Recently, cell-free/in vitro biosynthesis has emerged as a promising alternative to metabolic engineering of living cells for acetoin biomanufacturing due to its fast reaction rate, high product yield and stereoselectivity. However, it is still arduous to achieve an overall economically-feasible titer, rate and yield (TRY) during in vitro (3R)-acetoin biosynthesis. In this work, all known acetoin synthesis routes were analyzed, and the optimal α-acetolactate (α-AL) pathway was constructed for green and efficient enzymatic synthesis of (3R)-acetoin from pyruvate. α-Acetolactate synthetase (ALS) and α-acetolactate decarboxylase (ALDC) from Bacillus subtilis were selected from several candidates. After optimization of reaction conditions, 186.7 g L−1 (3R)-acetoin was obtained from 395.6 g L−1 pyruvate with 94.3% theoretical yield and 99.8% enantiomer excess at a rate of 15.56 g L−1 h−1. Finally, acetoin was isolated from the reaction system with a recovery of 70.58% and a purity of 98.24% through separation and purification. To the best of our knowledge, this is the first report on efficient biosynthesis, separation and purification of (3R)-acetoin in vitro with the highest titer and fastest average productivity ever reported. This work therefore provides a green and efficient alternative to biotechnological production of optically pure (3R)-acetoin.


 Please wait while we load your content...
Please wait while we load your content...