Praseodymium hydroxide/gold-supported precursor: a new strategy for preparing stable and active catalyst for the water-gas shift reaction†
Abstract
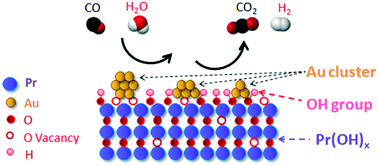
Praseodymium oxide with a mixed-valence state (Pr4+/Pr3+) has the highest O mobility among the rare earth oxides (REOs) and holds great promise in the field of heterogeneous catalysis. Herein, a rod-shaped praseodymium hydroxide (Pr(OH)x) support was obtained via hydrothermal method and used for the immobilization of Au nano-species by a modified deposition–precipitation (MDP) method. The resulting Au/Pr(OH)x_M catalyst was tested for water-gas shift reaction (WGSR) and CO oxidation and exhibited high activity and stability in both reactions with a TOF of 0.55 s−1 for WGSR, which is high compared with the reported ceria-support Au catalysts. HAADF-STEM measurements combined with the elemental composition distribution maps verified Au sub-nanospecies formed on the surface. The EXAFS and XPS studies demonstrated the transformation of the chemical state of Au from ionic Au (Au3+) to metallic Au (Au0) after the WGSR. The photoemission spectra of Pr 3d and O 1s indicate high number of O vacancies on Au/Pr(OH)x_M, which facilitate the H2O dissociation and contribute to the improved WGSR. This study shows that praseodymium hydroxide, as a hydroxyl- and O vacancy-rich support can promote the dispersion and stabilization of Au species, thus providing useful concepts for the design and preparation of active and stable catalysts for heterogeneous catalysis.


 Please wait while we load your content...
Please wait while we load your content...