Anodic molecular hydrogen formation on Ru and Cu electrodes†
Abstract
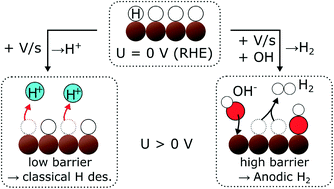
Electrochemical hydrogen adsorption and desorption, and the state of adsorbed hydrogen (*H) on metal surfaces are of fundamental interest as well as practical importance for the hydrogen evolution reaction (HER) and electrochemical hydrogenation reactions including CO2 and CO electroreduction. Here, we report a previously undiscovered phenomenon whereby *H desorbs as H2 during an anodic potential sweep at potentials anodic of (more positive than) the equilibrium potential of U0(H2/H+), hence at potentials where hydrogen desorption would be expected as H+. Using electrochemistry – online mass spectrometry, we observe, quantify, and characterize the phenomenon on two different materials in two different environments – Ru(0001) in acid and polycrystalline Cu in alkaline. For both Ru and Cu, the anodic H2 formation seems to coincide with *OH adsorption, which would be consistent with a displacement mechanism. We propose that a high barrier for the Volmer step causes some of the displaced *H to desorb as H2 (Tafel step) rather than the thermodynamically more favorable desorption as H+ (Volmer step).


 Please wait while we load your content...
Please wait while we load your content...