Non-oxidative dehydrogenation of isobutane over supported vanadium oxide: nature of the active sites and coke formation†
Abstract
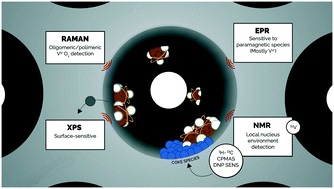
We combine Raman spectroscopy, electron paramagnetic resonance (EPR), X-ray photoelectron spectroscopy (XPS), temperature-programmed reduction (TPR), X-ray diffraction (XRD), high-field 51V-solid-state magic angle spinning NMR spectroscopy (ssNMR), transmission electron microscopy (TEM) and N2-physisorption to unravel structure–activity relationships during the non-oxidative dehydrogenation of isobutane over a V-based catalyst. The use of SBA-15 as a support favours the formation of oligomeric tetrahedral VOx species along with a smaller amount of V2O5 clusters. EPR, 51V-ssNMR and XPS suggest the formation of mostly V4+ species under reaction conditions. Investigation of “coke” species by dynamic nuclear polarization surface enhanced solid-state NMR (DNP SENS) reveals the co-existence of aliphatic, olefinic/aromatic, acetal/alkoxy and carbonyl-based organic moieties in the post-reacted catalyst. Together with TPR and XRD results, we postulate that oxygenated coke species are the main components responsible for vanadium clustering, which results in the irreversible deactivation of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...