The direct synthesis of hydrogen peroxide using a combination of a hydrophobic solvent and water†
Abstract
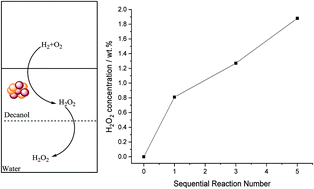
The direct synthesis of hydrogen peroxide (H2O2) has been studied using a solvent system comprising a hydrophobic alcohol (decan-1-ol) and water. It is demonstrated that, with the optimum combination of solvent and catalyst the contribution of H2O2 degradation pathways can be minimised to achieve industrially acceptable H2O2 concentrations under moderate conditions. This is achieved through the use of a catalyst that is retained by the organic component and the extraction of synthesised H2O2 into the aqueous phase, consequently limiting contact between the synthesised H2O2, catalyst and reactant gases, resulting in an improved selectivity towards H2O2. Investigation of the reaction parameters provides an insight into the proposed solvent system, and optimised conditions to produce H2O2 from molecular H2 and O2 have been identified. Through this optimisation H2O2 concentrations up to 1.9 wt% have been achieved via sequential gas replacement experiments.


 Please wait while we load your content...
Please wait while we load your content...