Oxidative dehydrogenation of propane over transition metal sulfides using sulfur as an alternative oxidant†
Abstract
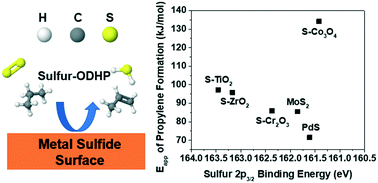
The use of alternative oxidants for the oxidative dehydrogenation of propane (ODHP) is a promising strategy to suppress the facile overoxidation to COx that occurs with O2. Gaseous disulfur (S2) represents a thermodynamically “softer” oxidant that has been underexplored and yet offers a potential route to more selective propylene formation. Here we describe a system for sulfur-ODHP (SODHP). We demonstrate that various metal sulfide catalysts generate unique reaction product distributions, and that propylene selectivities as high as 86% can be achieved at 450–550 °C. For a group of 6 metal sulfide catalysts, apparent activation energies for propylene formation range from 72–134 kJ mol−1 and parallel the corresponding catalyst XPS sulfur binding energies, indicating that M–S bond strength plays a key role in SODHP activity. Kinetic data over a sulfided ZrO2 catalyst indicate a rate law which is first-order in propane and zero-order in sulfur, suggesting that SODHP may occur via a mechanism analogous to the Mars van Krevelen cycle of traditional ODHP. The present results should motivate further studies of SODHP as a route to the selective and efficient oxidative production of propylene.


 Please wait while we load your content...
Please wait while we load your content...
