Role of Lewis and Brønsted acid sites in resorcinol tert-butylation over heteropolyacid-based catalysts†
Abstract
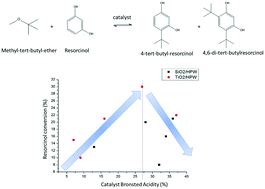
The role of Brønsted and Lewis acid sites at the surface of heteropolyacid-based catalysts was studied in resorcinol alkylation with methyl-tert-butylether. Three sets of catalysts, SiO2/HPW, TiO2/HPW and ZrO2/HPW (where HPW stands for phosphotungstic acid hydrate), synthesized by the hydrolytic sol–gel method were investigated. The surface total acidity was characterized by ammonia chemisorption and thermo-programmed desorption. In addition, infrared analysis of adsorbed pyridine was performed to distinguish between Brønsted and Lewis sites. The resorcinol conversion was correlated to the fraction of Brønsted sites present at the catalyst surface based on the total acidity. The results pointed out the importance of considering both Brønsted and Lewis sites as active players in the mechanism of resorcinol alkylation: Lewis sites have the role of adsorbing the substrate close to the tert-butyl cation, which is formed on Brønsted sites. Resorcinol conversion can be increased to a maximum if the right Brønsted/Lewis ratio is attained at the catalyst surface.


 Please wait while we load your content...
Please wait while we load your content...